In this article, we will explore the question “Is aluminium oxide ionic or covalent? Here are five interesting things to know about whether aluminium oxide is ionic or covalent:
1. Electronegativity Difference: Aluminium oxide (Al2O3) is an ionic compound due to the significant electronegativity difference between aluminum and oxygen, which is 2.0, indicating ionic bonding.
2. Composition: It comprises a metal (aluminum) and a nonmetal (oxygen), which is a characteristic of ionic compounds.
3. Polarizing Power: The ionic nature of aluminium oxide is influenced by the relative size of oxygen and aluminum and the polarizing power of the Al3+ ion.
4. Covalent Character: While it has ionic bonding, there is some added covalency due to the high charge density of the Al3+ ion, which distorts the electron cloud of the O2- ion, giving it added covalency.
5. Comparison with Sodium Oxide: Aluminium oxide acts less like an ionic compound and more like a covalent compound than sodium oxide due to the less ionic nature of the bond between aluminum and oxide ions.
These points provide a comprehensive understanding of the ionic and covalent nature of aluminium oxide, highlighting its ionic characteristics as well as the additional covalent aspects due to the specific properties of its constituent elements.
- Is aluminium oxide ionic or covalent? What are some properties of ionic compounds?
- How do the electronegativity differences between aluminum and oxygen in aluminium oxide contribute to its ionic nature?
- What is the charge of the aluminium ion in aluminium oxide?
- How does the high charge density of the Al3+ ion contribute to the added covalency in aluminium oxide?
- How do ionic compounds conduct electricity in their molten or dissolved state compared to their solid state?
- What are the factors that determine the stability of an ionic compound?
- What is the role of intermolecular forces in the stability of ionic compounds?
- How do intermolecular forces affect the melting and boiling points of ionic compounds?
- Helpful Resources
Is aluminium oxide ionic or covalent? What are some properties of ionic compounds?
Ionic compounds have several unique properties, including:
1. High Melting and Boiling Points: Due to the strong electrostatic forces holding the ions together, ionic compounds typically have high melting and boiling points.
2. Electrical Conductivity: Ionic compounds can conduct electricity in a molten or dissolved state, as their ions are free to move. However, they do not conduct electricity in their solid state.
3. Brittleness: They are typically hard and brittle, and when an ionic crystal breaks, it tends to do so along smooth planes because of the regular arrangement of the ions.
4. Crystal-like Structure: Ionic compounds have a crystal-like structure due to the regular and orderly arrangement of ions in the crystal lattice.
5. Insolubility in Nonpolar Solvents: Ionic compounds are generally insoluble in nonpolar solvents, but they can dissolve in polar solvents like water due to the attraction between the ions and the polar water molecules.
These properties are a result of the strong ionic bonds and the arrangement of ions in the crystal lattice structure of ionic compounds.
How do the electronegativity differences between aluminum and oxygen in aluminium oxide contribute to its ionic nature?
The electronegativity differences between aluminum and oxygen in aluminium oxide contribute to its ionic nature in the following ways:
1. Significant Electronegativity Difference: The electronegativity difference between aluminum (1.5) and oxygen (3.5) is 2.0, which is greater than the ionic bonding threshold of 1.8. This difference in electronegativity indicates that aluminum and oxygen atoms have a strong electrostatic attraction, leading to ionic bonding.
2. Electrostatic Attraction: The significant electronegativity difference between aluminum and oxygen results in strong electrostatic forces of attraction between them, which is characteristic of ionic bonding.
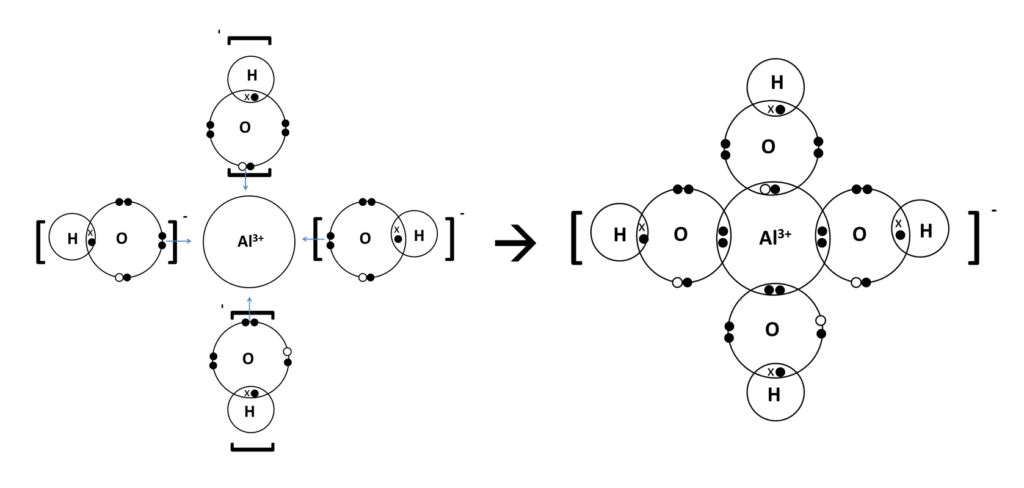
3. Formation of Ions: The electronegativity difference causes aluminum and oxygen atoms to form ions, with aluminum ions having a +3 charge and oxygen ions having a -2 charge. This arrangement of ions forms the characteristic ionic lattice of aluminium oxide.
4. High Charge Density: The high charge density of the Al3+ ion contributes to the ionic nature of aluminium oxide, as it can polarize the O2- ion, distorting its electron cloud and resulting in added covalency.
5. Regular Arrangement of Ions: The electronegativity differences between aluminum and oxygen lead to a regular and orderly arrangement of ions in the aluminium oxide crystal lattice, which is a characteristic property of ionic compounds.
In summary, the electronegativity differences between aluminum and oxygen in aluminium oxide contribute to its ionic nature by creating strong electrostatic forces of attraction between them, forming ions, and arranging themselves in a regular and orderly manner in the crystal lattice.
What is the charge of the aluminium ion in aluminium oxide?
In aluminium oxide (Al2O3), the aluminium ion has a +3 charge, while the oxygen ion has a -2 charge. This charge distribution results from the loss of electrons by aluminium atoms and the gain of electrons by oxygen atoms during the formation of ions, as follows:
1. Aluminium loses two electrons, resulting in a +3 charge (Al3+).
2. Oxygen gains two electrons, resulting in a -2 charge (O2-).
The +3 charge of the aluminium ion and the -2 charge of the oxygen ion create the ionic bonding in aluminium oxide, which is characteristic of ionic compounds.
How does the high charge density of the Al3+ ion contribute to the added covalency in aluminium oxide?
The high charge density of the Al3+ ion in aluminium oxide contributes to the added covalency in the following ways:
1. Polarization of the O2- Ion: The high charge density of the Al3+ ion, which is a result of its small atomic radius and high positive charge, polarizes the electron cloud of the O2- ion. This distortion of the electron cloud gives the bond some covalent character, in addition to its ionic nature.
2. Added Covalency: The polarization caused by the high charge density of the Al3+ ion results in the electron cloud of the O2- ion being more concentrated between the Al3+ and O2- ions than expected, giving aluminium oxide added covalency.
3. Comparison with Boron Compounds: The added covalency in aluminium oxide can be compared to the bonds in boron compounds, which are much more covalent due to the less metallic or more electronegative nature of boron. This highlights the influence of the high charge density of the Al3+ ion on the covalent character of the bond in aluminium oxide.
In summary, the high charge density of the Al3+ ion in aluminium oxide polarizes the electron cloud of the O2- ion, leading to added covalency in the bond between aluminum and oxygen ions. This results in aluminium oxide having some covalent character in addition to its ionic bonding.
How do ionic compounds conduct electricity in their molten or dissolved state compared to their solid state?
Ionic compounds conduct electricity in their molten or dissolved state, but not in their solid state. In the solid state, the ions are held in fixed positions within the crystal lattice and cannot move to conduct electricity.
However, when the ionic compound is melted or dissolved, the ions become free to move within the liquid or solution, allowing them to carry the electric charge and conduct electricity.
This change in electrical conductivity is due to the mobility of the ions in the molten or dissolved state, which is not possible in the solid state where the ions are held in a fixed position within the crystal lattice.
What are the factors that determine the stability of an ionic compound?
The stability of ionic compounds is determined by several factors, including:
1. Charge of Cations and Anions: The higher the charge of the cations and anions, the stronger the electrostatic forces of attraction between them, leading to increased stability.
2. Ease of Formation of Cations and Anions: This includes factors such as ionization enthalpy and bond dissociation energy. The easier it is to form cations and anions, the more stable the ionic compound.
3. Cation/Anion Size Ratio: The ratio of the sizes of the cations to the anions affects the lattice energy, which is a major determinant of stability. A proper size ratio leads to a more stable structure.
4. Lattice Point Arrangements: The arrangements of cations and anions in the most favorable lattice structure contribute to the stability of ionic compounds.
5. Ionic Bond Strength: The strength of the ionic bonds, which depends on the charge on the ions, influences the stability of the compound. Ions with higher charges have stronger forces between them, leading to increased stability.
6. Regular Arrangement of Ions: The regular and orderly arrangement of ions in the crystal lattice contributes to the stability of ionic compounds.
These factors collectively determine the stability of ionic compounds, influencing their physical and chemical properties, such as melting points, solubility, and conductivity.
What is the role of intermolecular forces in the stability of ionic compounds?
The role of intermolecular forces in the stability of ionic compounds is not significant. Intermolecular forces are the forces of attraction or repulsion that act between molecules.
They are much weaker than the intramolecular forces, such as ionic or covalent bonds. In the case of ionic compounds, the stability is primarily determined by factors such as the charge of the ions, the ease of formation of cations and anions, the cation/anion size ratio, and the lattice point arrangements.
These factors are responsible for the strong electrostatic forces of attraction between the ions in an ionic compound, which are much stronger than intermolecular forces. Therefore, intermolecular forces play a minor role in the stability of ionic compounds compared to the intramolecular forces that hold the compound together.
How do intermolecular forces affect the melting and boiling points of ionic compounds?
Ionic compounds have high melting and boiling points due to the strong electrostatic forces between the ions. Intermolecular forces, which are the forces between molecules, are much weaker than the intramolecular forces in ionic compounds.
Therefore, the role of intermolecular forces in the stability of ionic compounds is not significant. The strength of intermolecular forces does not have a direct impact on the melting and boiling points of ionic compounds.
Instead, the high melting and boiling points of ionic compounds are primarily attributed to the strong electrostatic forces of attraction between the ions, which are much stronger than intermolecular forces.
Helpful Resources
- https://study.com/academy/lesson/aluminum-oxide-formula-uses.html
- https://chemistry.stackexchange.com/questions/50122/how-is-al2cl6-covalent-and-al2o3-ionic
- https://www.answers.com/chemistry/Is_aluminium_oxide_ionic_or_covalent
- https://www.thestudentroom.co.uk/showthread.php?t=4322454
- https://studymind.co.uk/notes/ionic-compound-properties/
- https://www.bbc.co.uk/bitesize/guides/zyydng8/revision/4
- https://study.com/academy/lesson/ionic-compounds-formation-lattice-energy-and-properties.html
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Basics_of_General_Organic_and_Biological_Chemistry_(Ball_et_al.)/03:_Ionic_Bonding_and_Simple_Ionic_Compounds/3.06:__Characteristics_of_Ionic_Compounds
- https://edu.rsc.org/download?ac=521478
- https://www.echemi.com/community/why-does-aluminum-oxide-have-both-covalent-and-ionic-properties_mjart22040915906_66.html
- https://byjus.com/question-answer/ionic-solids-conduct-electricity-in-molten-state-but-not-in-solid-state-explain-1/
- https://www.mytutor.co.uk/answers/24892/GCSE/Chemistry/Why-do-ionic-compounds-conduct-electricity-when-molten-or-in-solution-but-not-when-solid/
- https://www.bbc.co.uk/bitesize/guides/z38smsg/revision/7
- https://www.echemi.com/community/what-factors-are-responsible-for-the-stability-of-ionic-compounds_mjart22041014930_125.html
- https://thecorrecter.com/can-aluminium-be-magnetised/
- https://thecorrecter.com/is-aluminium-monatomic-or-diatomic/
- https://thecorrecter.com/is-aluminium-toxicity-reversible/
- https://thecorrecter.com/what-to-do-if-you-accidentally-eat-aluminum-foil/
- https://thecorrecter.com/what-size-aluminium-wire-for-125-amp-service/
- https://thecorrecter.com/can-you-put-aluminium-foil-in-an-air-fryer/
- https://thecorrecter.com/how-to-remove-scratches-from-aluminium-4/
- https://thecorrecter.com/how-is-aluminium-mined-2/
- https://thecorrecter.com/is-aluminium-malleable-and-ductile/
- https://thecorrecter.com/does-aluminium-cause-dementia-2/
- https://thecorrecter.com/how-to-remove-anodising-from-aluminium-2/
- https://thecorrecter.com/how-to-restore-aluminium-boat/
- https://thecorrecter.com/how-does-aluminium-cans-affect-the-environment/
- https://thecorrecter.com/can-aluminium-be-soldered/
- https://thecorrecter.com/can-you-weld-aluminium-with-flux-core/
- https://thecorrecter.com/does-aluminium-foil-catch-fire/
- https://thecorrecter.com/how-to-clean-aluminium-siding/
- https://thecorrecter.com/can-aluminium-foil-be-recycled/
- https://thecorrecter.com/is-aluminium-conductive-to-lightning/
- https://thecorrecter.com/does-aluminium-foil-conduct-electricity/
- https://thecorrecter.com/can-you-weld-aluminium-with-a-stick-welder/
- https://thecorrecter.com/is-aluminium-metal-or-non-metal/
- https://thecorrecter.com/how-to-weld-aluminium-with-a-mig-welder/
- https://thecorrecter.com/how-to-solder-aluminium-with-a-soldering-iron-3-best-steps/
- https://thecorrecter.com/is-forged-aluminium-cookware-safe/
- https://thecorrecter.com/is-aluminium-in-deodorant-harmful/
