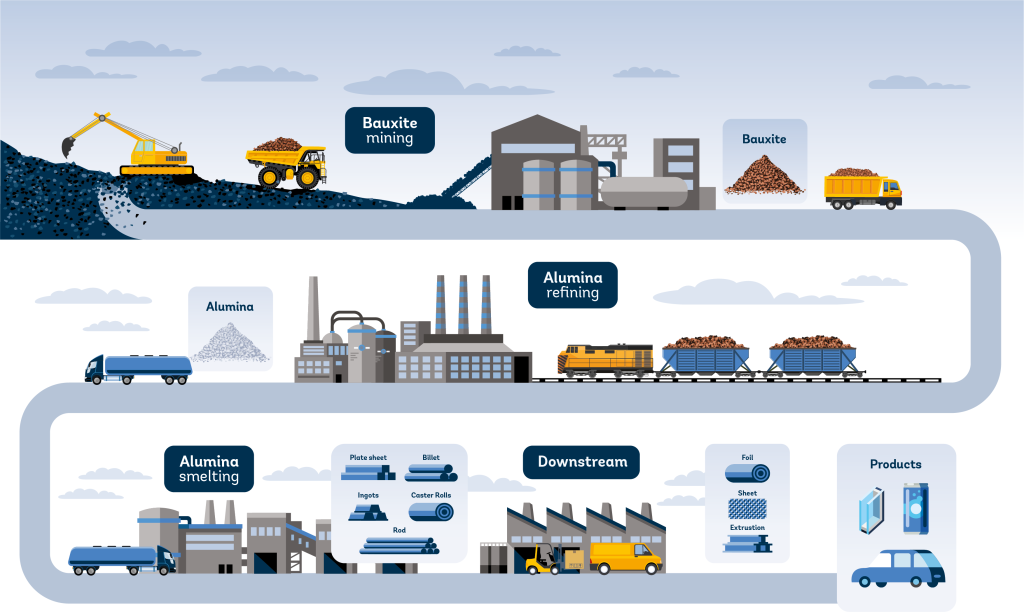
The process of mining aluminum involves several steps, from the extraction of bauxite ore to the production of pure aluminum. In this article, we will explore the question “How is Aluminium Mined?
- How is Aluminium Mined?
- How is bauxite extracted from the earth, and what are the primary methods used in the extraction process?
- What are the primary methods used to smelt alumina into aluminum?
- What are the primary uses of bauxite, aside from its role as the primary source of aluminum?
- What are the environmental impacts of bauxite mining?
- What are the primary methods used to recycle aluminum?
- Helpful Resources
How is Aluminium Mined?
Here is a detailed overview of how aluminum is mined:
1. Bauxite mining: The aluminum production process begins with the mining of bauxite, which is an aluminum-rich mineral found in the form of aluminum hydroxide. Bauxite is typically found in tropical areas, and about 90% of the global bauxite supply is located in these regions.
2. Alumina production: Once the bauxite is mined, it is crushed, dried, and ground in special mills, where it is mixed with a small amount of water. This process produces a thick paste that is collected in special containers and heated with steam to remove most of the silicon present in bauxite, resulting in the production of alumina, also known as aluminum oxide.
3. Reduction process: At an aluminum smelter, alumina is poured into special reduction cells with molten cryolite at 950°C. Electric currents are then induced in the mixture at 400 kA or above; this current breaks the bond between the aluminum and oxygen atoms, resulting in liquid aluminum settling at the bottom of the reduction cell.
4. Primary aluminum production: The liquid aluminum produced in the reduction cells is cast into ingots and shipped to customers or used in the production of aluminum alloys for various purposes.
5. Aluminum alloys: The primary aluminum is shaped into its required form, which is used for making the vast majority of aluminum products, from spectacle frames to telephone bodies, aeroplane fuselages, or spaceship bodies.
6. Recycling: Unlike iron, aluminum is corrosion-resistant, so it can be remelted and reused an infinite number of times. The added benefit is that recycling aluminum requires only 5% of the energy needed to make the same amount of primary aluminum.
In summary, the process of mining aluminum involves the extraction of bauxite ore, the production of alumina, the reduction of alumina to produce pure aluminum, and the shaping of aluminum into its required form. This process enables the production of a wide range of aluminum products for various industries.
How is bauxite extracted from the earth, and what are the primary methods used in the extraction process?
The extraction of bauxite, the primary ore of aluminum, involves several key steps. Here are the primary methods used in the extraction process:
1. Mining: Bauxite is generally extracted by open-cast mining, as it is almost always found near the surface. The mining process involves the clearing of land, removal of topsoil, and the use of methods such as blasting, drilling, and ripping with large bulldozers to break up the bauxite layer.
2. Crushing and grinding: Once the bauxite is loosened, it is loaded into trucks, railroad cars, or conveyors and transported to crushing and washing plants or stockpiles. The bauxite ore is washed and crushed, reducing the particle size and increasing the available surface area for the digestion stage.
3. Leaching: The bauxite ore is then leached with caustic soda (NaOH) to produce soluble sodium aluminate (NaAlO2), leaving the impurities in the insoluble residue. This process is known as the Bayer Process and is the most economic means of obtaining alumina from bauxite.
4. Digestion: The leached bauxite is then subjected to digestion, where conditions such as caustic concentration, temperature, and pressure are set according to the properties of the bauxite ore. This stage results in the dissolution of alumina from the bauxite ore to form sodium aluminate.
5. Clarification and precipitation: The first stage of clarification is to separate the solids (bauxite residue) from the pregnant liquor (sodium aluminate solution). The clarified solution is then seeded with fine alumina crystals to encourage the precipitation of pure alumina hydrate, which is then separated and washed to remove impurities.
In summary, the extraction of bauxite from the earth involves open-cast mining, crushing, grinding, leaching, digestion, and clarification processes to obtain alumina, the precursor to aluminum. These processes are essential for the production of aluminum, which is used in a wide range of applications.
What are the primary methods used to smelt alumina into aluminum?
The primary method used to smelt alumina into aluminum is the Hall-Héroult process. This process involves the electrolytic reduction of alumina (aluminum oxide) to produce aluminum metal. Here are the key steps involved in the Hall-Héroult process:
1. Alumina production: The process begins with the production of alumina from bauxite ore through the Bayer process. This involves the extraction of alumina from bauxite ore using caustic soda, followed by the refining and purification of the alumina to produce a white powder.
2. Electrolysis: The purified alumina is then dissolved in molten cryolite, a fluoride mineral, and placed in an electrolytic cell. The cell is made of a steel shell with a series of insulating linings of refractory material. Carbon anodes are immersed in the molten alumina, and a high current is passed through the cell.
3. Reduction: The high current passing through the cell causes the alumina to decompose, releasing oxygen at the carbon anodes and producing molten aluminum at the cathode. The molten aluminum collects at the bottom of the cell and is periodically siphoned off.
4. Casting: The molten aluminum is then cast into ingots or other desired shapes for further processing and use.
The Hall-Héroult process is the primary method used to produce primary aluminum, and it is known for its high energy consumption and carbon emissions. However, it remains the most widely used method for aluminum production due to its efficiency and cost-effectiveness.
What are the primary uses of bauxite, aside from its role as the primary source of aluminum?
Apart from being the primary source of aluminum, bauxite has several other industrial uses. Some of the main uses of bauxite include:
1. Cement production: Bauxite is used as a raw material for producing cement, which is a crucial building material used in construction.
2. Metallurgy: Bauxite is used in various processes like the Bayer Process and Hall-Heroult Process for the production of aluminum and aluminum-based alloys, which are widely used in electronics, construction, and utensils.
3. Chemical industry: Bauxite is used in the manufacturing of aluminum chemicals, which are used in various applications, such as detergents, cosmetics, and pharmaceuticals.
4. Refractory materials: Bauxite is used as a raw material for producing refractory materials, which are used in the construction of furnaces, kilns, and reactors.
5. Abrasives: Bauxite is used in the production of abrasives, such as grinding wheels and polishing tools, due to its hardness and abrasive properties.
6. Cement production: Bauxite is used in the production of cement, which is a widely used building material.
7. Desiccating agent, adsorbent, and catalyst: Bauxite is used as a desiccating agent, adsorbent, and catalyst in various applications, such as the purification of chemicals and the production of dental cement.
In summary, bauxite, apart from being the primary source of aluminum, has several other industrial uses, including cement production, metallurgy, chemical industry, refractory materials, abrasives, and as a desiccating agent, adsorbent, and catalyst.
What are the environmental impacts of bauxite mining?
Bauxite mining has several environmental impacts, which can affect the surrounding ecosystems and communities. Here are some of the primary environmental impacts of bauxite mining:
1. Deforestation: Bauxite mining often involves the clearing of large areas of forest, which can lead to habitat loss and biodiversity decline. This can have long-term impacts on the ecosystem and the species that depend on it.
2. Soil erosion: The removal of topsoil during bauxite mining can lead to soil erosion, which can cause sedimentation in nearby water bodies, affecting water quality and aquatic life.
3. Water pollution: Bauxite mining can lead to water pollution, as the chemicals used in the mining process can contaminate nearby water sources, affecting the health of both humans and wildlife.
4. Air pollution: Bauxite mining can also lead to air pollution, as the dust and particulate matter generated during the mining process can affect air quality and human health.
5. Climate change: The energy-intensive process of refining bauxite into alumina and aluminum can contribute to greenhouse gas emissions, which can contribute to climate change.
7. Community displacement: Bauxite mining can lead to the displacement of local communities, as mining operations often require the relocation of people living in the area. This can have social and economic impacts on the affected communities.
In summary, bauxite mining has several environmental impacts, including deforestation, soil erosion, water and air pollution, climate change, and community displacement.
These impacts can have long-term effects on the surrounding ecosystems and communities, highlighting the need for responsible and sustainable mining practices.
What are the primary methods used to recycle aluminum?
The primary methods used to recycle aluminum include:
1. Collection and sorting: The first step in aluminum recycling is the collection and sorting of aluminum scrap from various sources. Scrap is sorted based on alloy type, grade, impurity levels, and other factors. Proper sorting is essential for producing high-quality recycled aluminum.
2. Pre-treatment: Once collected, aluminum scrap is pre-treated to remove any contaminants, such as paint, coatings, or other metals. This may involve shredding, crushing, or melting the scrap to prepare it for the recycling process.
3. Melting and re-melting: The pre-treated aluminum scrap is then melted in a furnace to produce molten aluminum. This molten aluminum can be used to produce new aluminum products, such as ingots, sheets, or extrusions. The re-melting of aluminum scrap is cheaper and more energy-efficient than the production of aluminum from raw bauxite via electrolysis.
4. Casting and fabrication: The molten aluminum produced from recycled scrap can be cast into ingots or other desired shapes for further processing and use. It can also be used to produce new aluminum products through processes such as extrusion, rolling, or forging.
In summary, the primary methods used to recycle aluminum include collection and sorting, pre-treatment, melting and re-melting, and casting and fabrication.
Recycling aluminum is both economically and environmentally effective, as recycled aluminum requires only 5% of the energy used to make primary aluminum, and can have the same properties as the parent metal.
Helpful Resources
- https://aluminiumleader.com/production/aluminum_production/
- https://bauxite.world-aluminium.org/mining/process/
- https://bauxite.world-aluminium.org/refining/process/
- https://www.911metallurgist.com/blog/extraction-process-of-aluminium-from-bauxite-ore
- https://en.wikipedia.org/wiki/Aluminium_smelting
- https://www.sciencedirect.com/topics/earth-and-planetary-sciences/bauxite
- https://byjus.com/chemistry/uses-of-bauxite/
- https://www.cmswire.com/digital-experience/how-bauxite-mining-destroys-nature-and-communities/
- https://www.sciencedirect.com/science/article/abs/pii/S0959652622052945
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4934713/
- https://www.thesca.org/connect/blog/environmental-impact-aluminum/
- https://en.wikipedia.org/wiki/Aluminium_recycling
- https://thecorrecter.com/how-to-remove-anodising-from-aluminium-2/
- https://thecorrecter.com/how-to-restore-aluminium-boat/
- https://thecorrecter.com/how-does-aluminium-cans-affect-the-environment/
- https://thecorrecter.com/can-aluminium-be-soldered/
- https://thecorrecter.com/can-you-weld-aluminium-with-flux-core/
- https://thecorrecter.com/does-aluminium-foil-catch-fire/
- https://thecorrecter.com/can-aluminium-foil-be-recycled/
- https://thecorrecter.com/how-to-clean-aluminium-siding/
- https://thecorrecter.com/is-aluminium-conductive-to-lightning/
- https://thecorrecter.com/does-aluminium-foil-conduct-electricity/
- https://thecorrecter.com/is-aluminium-metal-or-non-metal/
- https://thecorrecter.com/can-you-weld-aluminium-with-a-stick-welder/
- https://thecorrecter.com/how-to-solder-aluminium-with-a-soldering-iron-3-best-steps/
- https://thecorrecter.com/is-aluminium-in-deodorant-harmful/
- https://thecorrecter.com/how-to-weld-aluminium-with-a-mig-welder/
- https://thecorrecter.com/is-forged-aluminium-cookware-safe/
