Monatomic elements consist of single atoms, while diatomic elements consist of molecules with two atoms. The distinction between monatomic and diatomic elements is essential for understanding the properties and behavior of various chemical elements.
In this article, we will explore the question “Is aluminium monatomic or diatomic?
- Is aluminium monatomic or diatomic?
- How are monatomic elements different from diatomic elements?
- How was the diatomic nature of many common gaseous elements originally determined?
- Why do some elements exist as diatomic molecules?
- How do diatomic elements differ from polyatomic elements?
- What are some common uses of diatomic elements in industry?
- What are some common uses of Monoatomic elements in industry?
- How are monatomic elements used in the production of semiconductors?
- What are some advantages of using monatomic semiconductors in electronic devices?
- Helpful Resources
Is aluminium monatomic or diatomic?
Here are ten things to know:
1. Aluminium is a monatomic element, meaning it is composed of a single atom.
2. Monatomic elements, like aluminium, exist as individual atoms without any bonds with other atoms.
3. In contrast, diatomic elements consist of molecules with two atoms bonded together.
4. Diatomic molecules can be of the same or different chemical elements.
5. Hundreds of diatomic molecules have been identified in the environment and in the laboratory.
6. Diatomic elements played an important role in the elucidation of the concepts of element, atom, and molecule in the 19th century.
7. Some common diatomic elements include hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), chlorine (Cl2), and bromine (Br2).
8. The chemical formula for aluminium is Al, as it is a monatomic element.
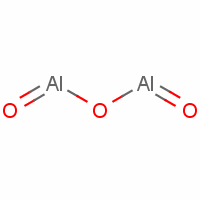
9. Aluminium oxide (Al2O3) is an example of a diatomic compound, as it consists of two aluminium atoms and three oxygen atoms.
10. The distinction between monatomic and diatomic elements is essential for understanding the properties and behavior of various chemical elements.
How are monatomic elements different from diatomic elements?
Monatomic elements and diatomic elements differ in the number of atoms they consist of and the way they bond with other elements. Here are the key differences between them:
1. Monatomic elements have a single atom, while diatomic elements have two atoms bonded together.
2. Monatomic elements exist as individual atoms without any bonds with other atoms.
3. Diatomic elements can be of the same or different chemical elements.
4. Monatomic elements are generally less reactive, while diatomic elements can be involved in various chemical reactions.
5. Noble gases, such as helium, neon, and argon, are examples of monatomic elements.
6. Diatomic molecules can be homonuclear (composed of the same element) or heteronuclear (composed of two different elements).
7. Diatomic elements played an important role in the elucidation of the concepts of element, atom, and molecule in the 19th century.
8. Hundreds of diatomic molecules have been identified in the environment and in the laboratory.
9. Monatomic elements are rarely found in their pure singular form, as they tend to bond with other elements.
10. The distinction between monatomic and diatomic elements is essential for understanding the properties and behavior of various chemical elements.
How was the diatomic nature of many common gaseous elements originally determined?
The diatomic nature of many common gaseous elements was originally determined through various methods. One of the earliest methods was through the observation of the physical properties of gases.
For example, the gases hydrogen, nitrogen, oxygen, fluorine, chlorine, and bromine were found to have similar physical properties, such as boiling points and densities, which suggested that they were composed of diatomic molecules.
Another method was through the study of chemical reactions involving these gases. For instance, the reaction of hydrogen and oxygen to form water was found to require two hydrogen atoms and one oxygen atom, indicating that both elements existed as diatomic molecules.
Additionally, spectroscopic techniques, such as infrared and Raman spectroscopy, have been used to study the vibrational and rotational modes of diatomic molecules.
These methods have allowed scientists to determine the bond lengths, bond energies, and other properties of diatomic molecules, which have contributed to our understanding of chemical bonding and molecular structure.
Why do some elements exist as diatomic molecules?
Some elements exist as diatomic molecules due to the need for increased stability. The diatomic nature of certain elements allows them to follow the octet rule, which states that atoms are most stable when they have a full outer electron shell.
By forming diatomic molecules, these elements can achieve a full outer shell and become more stable. For example, in the case of chlorine, which has seven electrons in its outer shell, it forms a diatomic molecule (Cl2) by sharing electrons with another chlorine atom, thereby achieving a more stable configuration.
The diatomic nature of elements like oxygen and nitrogen also contributes to their increased stability, making them more prevalent in the Earth’s atmosphere.
Therefore, the formation of diatomic molecules is a way for certain elements to achieve greater stability, which is a fundamental principle in chemical bonding and the behavior of elements.
How do diatomic elements differ from polyatomic elements?
Diatomic elements differ from polyatomic elements in the number of atoms they consist of and the way they bond. Here are the key differences:
1. Diatomic elements consist of two atoms of the same element bonded together, such as H2, N2, O2, F2, Cl2, Br2, and I2.
2. Polyatomic elements, on the other hand, consist of more than two atoms of the same element bonded together. For example, phosphorus (P4) and sulfur (S8) are polyatomic elements.
3. Diatomic elements are also referred to as homonuclear diatomic molecules, as both atoms in the molecule are the same.
4. Polyatomic elements are more complex, consisting of a larger number of atoms of the same element bonded together, which can form various structures such as rings or chains.
5. Diatomic elements, such as hydrogen and oxygen, are commonly found in the gaseous state at room temperature, while polyatomic elements like phosphorus and sulfur have different physical properties due to their larger molecular structures.
In summary, diatomic elements consist of two atoms of the same element bonded together, while polyatomic elements consist of more than two atoms of the same element bonded together, leading to differences in their chemical and physical properties.
What are some common uses of diatomic elements in industry?
The common uses of diatomic elements in industry are as follows:
1. Hydrogen (H2) is used in the production of ammonia for fertilizers, in the hydrogenation of vegetable oils, and in the production of methanol.
2. Nitrogen (N2) is used in the production of ammonia, in the food and beverage industry for packaging and preservation, and in the electronics industry for manufacturing semiconductors.
3. Oxygen (O2) is used in steelmaking, metal cutting and welding, water treatment, and in the production of chemicals and pharmaceuticals.
4. Fluorine (F2) is used in the production of uranium hexafluoride for the nuclear industry, in the production of Teflon and other fluoropolymers, and in the manufacturing of high-octane fuels.
5. Chlorine (Cl2) is used in the production of PVC, in water treatment, and in the manufacturing of various chemicals such as solvents, pesticides, and pharmaceuticals.
6. Iodine (I2) is used in the production of disinfectants, in the manufacturing of LCD displays, and in the production of pharmaceuticals and dyes.
7. Bromine (Br2) is used in the production of flame retardants, in the manufacturing of pharmaceuticals, and in the oil and gas industry for drilling fluids.
These diatomic elements have a wide range of industrial applications due to their unique chemical properties and reactivity.
What are some common uses of Monoatomic elements in industry?
Some common uses of monatomic elements in industry are as follows:
1. Helium (He) is used in the production of high-pressure gases, as a shielding gas in particle accelerators, and in the electronics industry for semiconductors.
2. Neon (Ne) is used in neon lighting, as a filler gas for incandescent light bulbs, and in the production of high-performance light bulbs.
3. Argon (Ar) is used in the production of silicon for the semiconductor industry, as a shielding gas in welding processes, and in the electronics industry for semiconductors.
4. Krypton (Kr) and xenon (Xe) are used in high-performance light bulbs, which have higher color temperatures and greater efficiency.
5. Xenon is also used as an an anesthetic due to its high solubility in lipids, which makes it effective for total body relaxation during medical procedures.
These monatomic elements have various industrial applications due to their unique properties and reactivity.
How are monatomic elements used in the production of semiconductors?
Monatomic elements, such as germanium and silicon, are used in the production of semiconductors. Semiconductors are essential materials in microelectronics and other fields, and electronic switching elements such as diodes or transistors are based on them.
The monatomic nature of these elements allows them to have unique electronic properties, which make them useful in the production of semiconductors.
Additionally, monatomic layers of precious metals, such as gold and silver, have been found to become semiconductors, which could have potential applications in the electronics industry.
Therefore, the unique electronic properties of monatomic elements make them useful in the production of semiconductors, which are essential components in modern electronics.
What are some advantages of using monatomic semiconductors in electronic devices?
Some advantages of using monatomic semiconductors in electronic devices include:
1. High sensitivity: Monatomic semiconductors, such as germanium and silicon, have been found to be extremely useful for sensing light energy and electrical signals due to their high sensitivity.
2. Low power consumption: These semiconductors can generate, amplify, and modulate electrical signals with low power consumption, making them ideal for various applications in electronics.
3. High electrical resistance: Monatomic semiconductors, such as gold and silver, can exhibit high electrical resistance, which can be beneficial in certain electronic applications.
4. Two-dimensional properties: Monatomic layers of materials like gold and silver can exhibit unique two-dimensional properties, potentially leading to applications in microelectronics and sensor technology.
5. Bandgap tunability: By combining different elements, the bandgap of monatomic semiconductors can be adjusted, allowing for the development of semiconductors with desired properties.
Overall, monatomic semiconductors offer advantages in terms of sensitivity, low power consumption, high electrical resistance, two-dimensional properties, and bandgap tunability, making them valuable components in electronic devices.
Helpful Resources
- https://byjus.com/physics/monatomic-gases/
- https://www.thoughtco.com/what-are-the-seven-diatomic-elements-606623
- https://en.wikipedia.org/wiki/Diatomic_molecule
- https://www.thoughtco.com/monatomic-or-monoatomic-elements-606630
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Basics_of_General_Organic_and_Biological_Chemistry_(Ball_et_al.)/02:_Elements_Atoms_and_the_Periodic_Table/2.02:_Atomic_Theory
- https://www.chemicalforums.com/index.php?topic=21640.15
- https://www.differencebetween.com/difference-between-monatomic-and-vs-diatomic/
- https://chemistry.stackexchange.com/questions/22270/how-was-the-diatomic-nature-of-many-common-gaseous-elements-originally-determine
- https://chemistrytalk.org/diatomic-elements/
- https://learnwithdrscott.com/diatomic-elements/
- https://youtube.com/watch?v=uvAI4-5T_zg
- https://study.com/academy/lesson/what-is-a-diatomic-element-definition-examples.html
- https://en.wikipedia.org/wiki/Noble_gas
- https://patents.google.com/patent/US2743201
- https://chem.libretexts.org/Courses/City_College_of_San_Francisco/CCSF_Chemistry_Resources/03:_CHE_202_-_General_Chemistry_II/3.08:_Semiconductors/3.8.02:_Structure_and_General_Properties_of_the_Metalloids
- https://www.mpg.de/14903250/gold-silver-layer-twodimension-semiconductor
- https://patents.google.com/patent/US2818536A/en
- https://thecorrecter.com/what-to-do-if-you-accidentally-eat-aluminum-foil/
- https://thecorrecter.com/can-you-put-aluminium-foil-in-an-air-fryer/
- https://thecorrecter.com/what-size-aluminium-wire-for-125-amp-service/
- https://thecorrecter.com/how-to-remove-scratches-from-aluminium-4/
- https://thecorrecter.com/is-aluminium-malleable-and-ductile/
- https://thecorrecter.com/how-is-aluminium-mined-2/
- https://thecorrecter.com/how-to-remove-anodising-from-aluminium-2/
- https://thecorrecter.com/does-aluminium-cause-dementia-2/
- https://thecorrecter.com/how-to-restore-aluminium-boat/
- https://thecorrecter.com/how-does-aluminium-cans-affect-the-environment/
- https://thecorrecter.com/can-aluminium-be-soldered/
- https://thecorrecter.com/does-aluminium-foil-catch-fire/
- https://thecorrecter.com/how-to-clean-aluminium-siding/
- https://thecorrecter.com/can-you-weld-aluminium-with-flux-core/
- https://thecorrecter.com/can-aluminium-foil-be-recycled/
- https://thecorrecter.com/does-aluminium-foil-conduct-electricity/
- https://thecorrecter.com/is-aluminium-conductive-to-lightning/
- https://thecorrecter.com/can-you-weld-aluminium-with-a-stick-welder/
- https://thecorrecter.com/how-to-weld-aluminium-with-a-mig-welder/
- https://thecorrecter.com/is-aluminium-metal-or-non-metal/
- https://thecorrecter.com/how-to-solder-aluminium-with-a-soldering-iron-3-best-steps/
- https://thecorrecter.com/is-forged-aluminium-cookware-safe/
- https://thecorrecter.com/is-aluminium-in-deodorant-harmful/
